Our group’s research and technology development has two main thrusts. The first is ultraviolet-excited fluorescence microscopy, a new technique for rapid multicolour tissue imaging. The second is optical coherence tomography, a timeless and versatile approach to non-invasive label-free imaging of tissue structure. Our hardware efforts are further enriched by data-driven learning for rapid machine interpretation and automated resolution enhancement.
Ultraviolet microscopy
Ultraviolet light in the sub-300nm range is well known for disinfection but has powerful unexplored potential for bioimaging. We developed a unique approach to ultraviolet-excited fluorescence to achieve rapid high-resolution imaging from bulk unsectioned tissue, in a 30 x 30 cm footprint and <5 min procedure. Energetic pursuit of new applications from surgical guidance (in collaboration with National University Hospital) to fundamental biology (A*STAR, National University of Singapore) is ongoing.

Joel Lang Yi Ang*, Ko Hui Tan*, Alexander Si Kai Yong*, Chiyo Wan Xuan Tan, Jessica Sze Jia Kng, Cyrus Jia Jun Tan, Rachael Hui Kie Soh, Julian Yi Hong Tan, and Kaicheng Liang, “Multi-scale tissue fluorescence mapping with fiber optic ultraviolet excitation and generative modeling,” Optica, 2024.
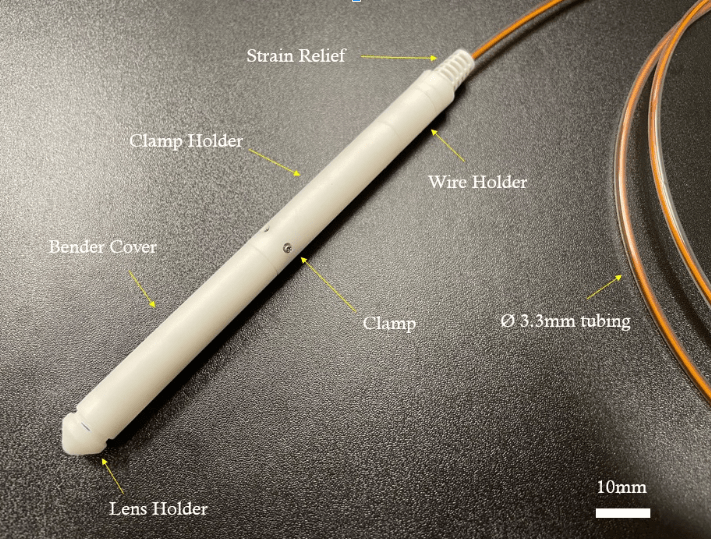
OCT applications and new technology
Optical coherence tomography is found in every eye clinic in the world, but OCT continues to capture the imagination in countless other fields. We invented a new way to perform OCT scanning 100X cheaper with no cost to performance, enabling new surgical imaging applications (in collaboration with National Neuroscience Institute). We are also developing methods to assess 3D cell culture morphology and activity with non-destructive dynamic OCT.
Rachel Yixuan Tan, Rachel Chi Kei Chan, Whitney Jia Ying Loh, Kaicheng Liang, “Miniaturized 2D Scanning Microscopy with a Single 1D Actuation for Multibeam Optical Coherence Tomography,” ACS Photonics, 2023.
Ko Hui Tan*, Joel Lang Yi Ang*, Alexander Si Kai Yong, Stefanie Zi En Lim, Jessica Sze Jia Kng, Kaicheng Liang, “Non-destructive viability assessment of cancer cell spheroids using dynamic optical coherence tomography with trypan blue validation,” Biomedical Optics Express, 2024.
Previous work
Precision microscanning
Tissue microscopy needs precision scanning, which is difficult to achieve in a small, minimally invasive package. We employ micro-actuators and miniaturized designs to realize 2-dimensional laser scanning in side-view or forward-view, compatible with any flying-spot microscopy.
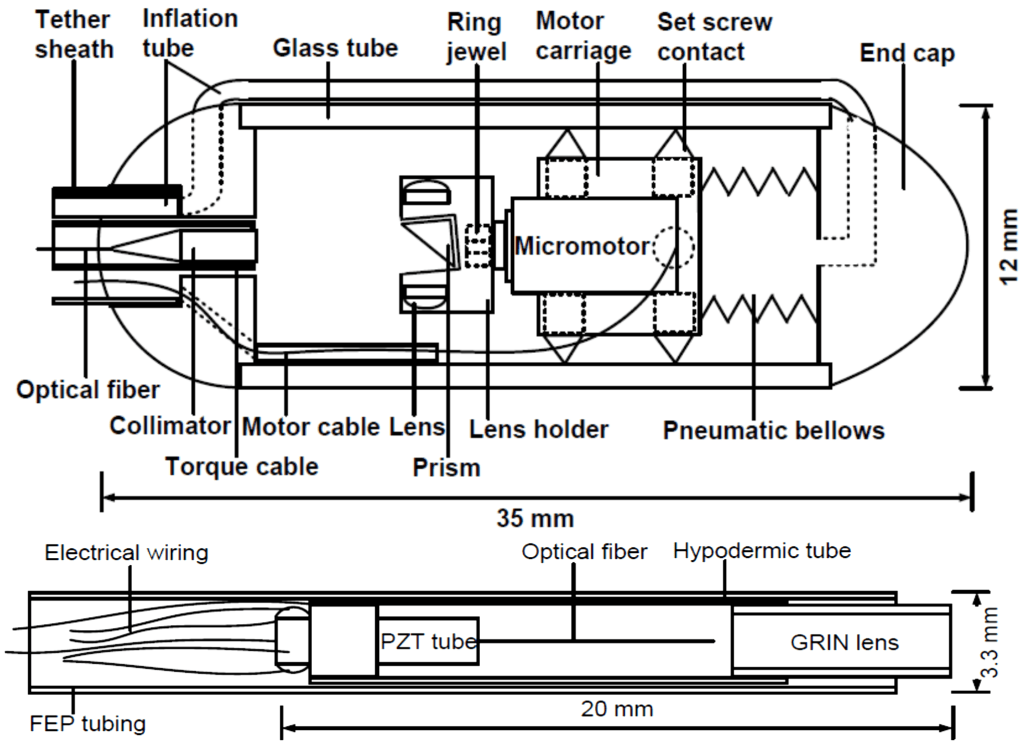
K. Liang*, Z. Wang*, O.O. Ahsen, H.-C. Lee, B.M. Potsaid, V. Jayaraman, A. Cable, H. Mashimo, X. Li, and J.G. Fujimoto, “Cycloid scanning for wide field optical coherence tomography endomicroscopy and angiography in vivo,” Optica 5, 36-43 (2018)
K. Liang, O.O. Ahsen, Z. Wang, H.-C. Lee, W. Liang, B.M. Potsaid, T.-H. Tsai, M.G. Giacomelli, V. Jayaraman, H. Mashimo, X. Li, and J.G. Fujimoto, “Endoscopic forward-viewing optical coherence tomography and angiography with MHz swept source,” Opt. Lett. 42, 3193-3196 (2017)
K. Liang, G. Traverso, H.-C. Lee, O.O. Ahsen, Z. Wang, B. Potsaid, M. Giacomelli, V. Jayaraman, R. Barman, A. Cable, H. Mashimo, R. Langer, and J.G. Fujimoto, “Ultrahigh speed en face OCT capsule for endoscopic imaging,” Biomed. Opt. Express 6, 1146-1163 (2015)
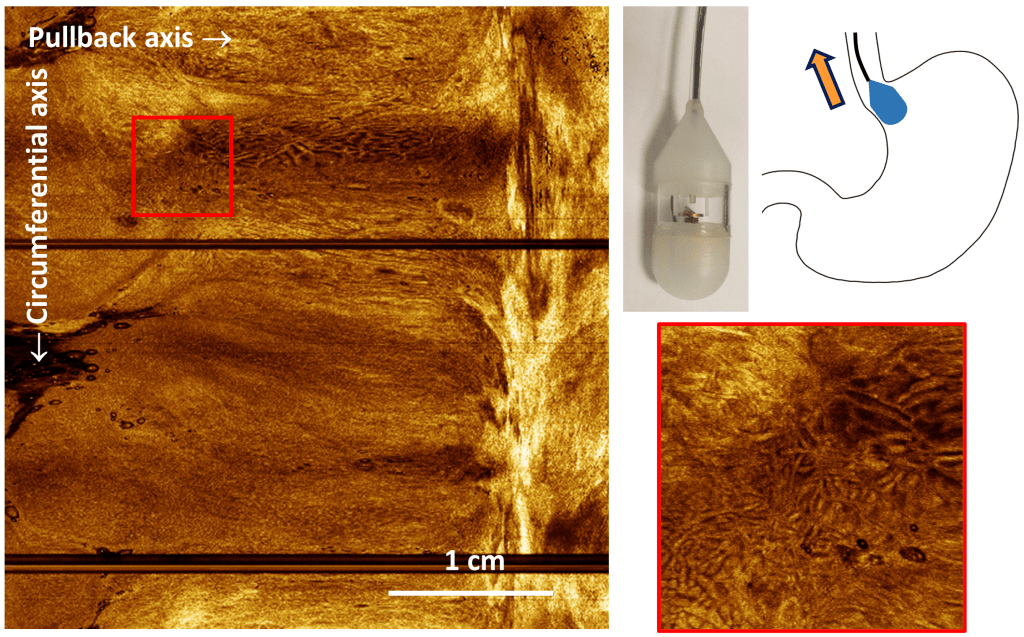
High speed endoscopic microscopy
Endoscopy is complex and requires anesthesia. Diagnosis requires microscopic assessment of the organ surface. Ultrafast Optical Coherence Tomography at millions of axial scans/sec, combined with a micromotor scanning tethered capsule, delivers gigantic fields-of-view in the human esophagus without needing anesthesia.
K. Liang, O.O. Ahsen, A. Murphy, J. Zhang, T.H. Nguyen, B.M. Potsaid, M. Figueiredo, Q. Huang, H. Mashimo, and J.G. Fujimoto, “Tethered capsule en face optical coherence tomography for imaging Barrett’s oesophagus in unsedated patients,” BMJ Open Gastroenterology 7 (2020)
K. Liang, O.O. Ahsen, H.-C. Lee, Z. Wang, B.M. Potsaid, M. Figueiredo, V. Jayaraman, A. Cable, Q. Huang, H. Mashimo, and J.G. Fujimoto, “Volumetric mapping of Barrett’s esophagus and dysplasia with en face Optical Coherence Tomography tethered capsule,” American Journal of Gastroenterology 111 (2016)
Computational imaging with AI
The optical resolution and imaging performance of endoscopic microscopy can be limited by size contraints of optical components. Neural networks can be trained to learn from high resolution images, to computationally enhance low resolution data.
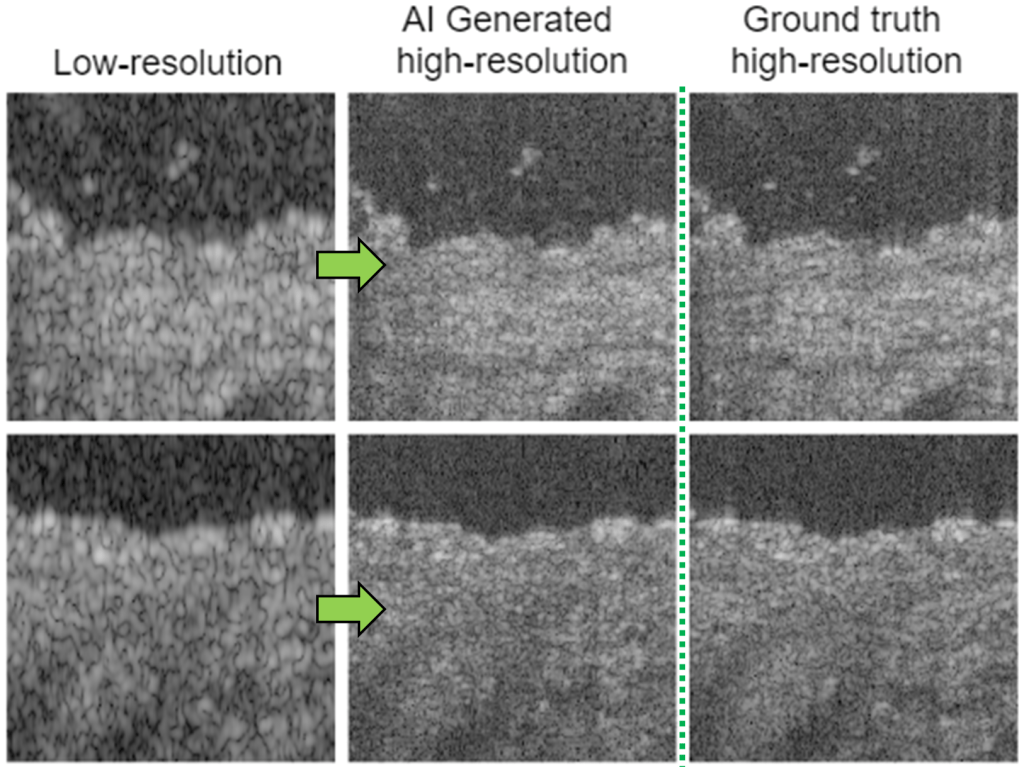
K. Liang, X. Liu, S. Chen, J. Xie, W.Q. Lee, L. Liu, H.K. Lee, “Resolution enhancement and realistic speckle recovery with generative adversarial modeling of micro-optical coherence tomography,” Biomed. Opt. Express (2020)
